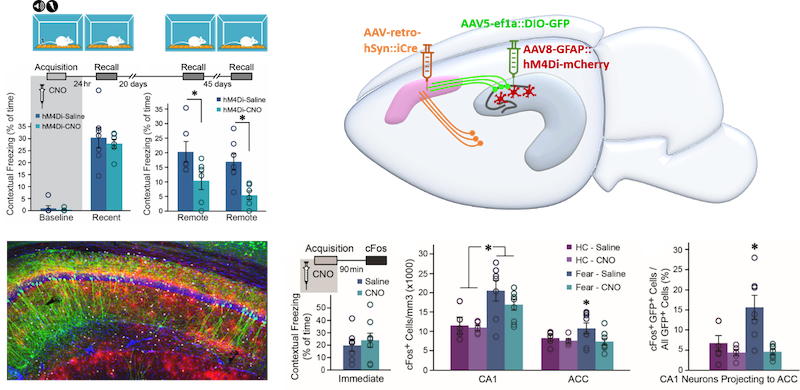
Remote memories depend on the coordinated activity in the hippocampus and frontal cortices, but the timeline of these interactions is debated. Astrocytes sense and modify neuronal activity, but their role in remote memory is not explored. We manipulated astrocytic activity in the CA1 region of the hippocampus during acquisition and discovered it specifically impaired remote, but not recent, memory recall. Moreover, we revealed a massive recruitment of anterior cingulate cortex (ACC)-projecting CA1 neurons, a process that was specifically inhibited by astrocytic manipulation, during memory acquisition. Finally, direct inhibition of CA1-to-ACC-projecting neurons spared recent and impaired remote memory. So far, it was assumed that remote memory is the product of recent memory. However, our findings show that the foundation of remote memory can be independently established during acquisition and that it involves projection-specific functions of astrocytes in regulating CA1-to-ACC neuronal communication.