The highly addictive drug Fentanyl is a powerful synthetic opioid that has been linked to a sharp increase in overdose deaths worldwide: In 2021, fentanyl was responsible for over 100,000 deaths in the United States alone. The social environment of individuals is known to have a crucial impact on the development of drug addiction, such that exposure to peers who consume drugs can increase drug use, while healthy social interactions can contribute to the success of different intervention programs.
Insight into the neural circuit basis of drug addiction depends on models of drug consumption. However, until now, rodent models largely ignored the social aspects of addiction, and could not account for social influence on drug consumption. In our work, we established a behavioral paradigm in rodents amenable for exploration of addictive behaviors in mice that maintain their natural social setting. We implemented this paradigm for investigation of the neural circuit basis modulating self-administration of fentanyl.
The solution we developed, is that of an behavioral measurement system called HOMECAGE, in which mice volitionally consume the opioid drug fentanyl in their home cage while maintaining social interactions. This setup, which runs without the need for experimenter intervention, can monitor the behavior of multiple mice continuously, yielding high-resolution behavioral data. Here, we use HOMECAGE to compare opioid consumption in mice belonging to an experimental group (in which we inhibited the activity of neurons in a brain region called the claustrum) and co-housed control mice. We observed that the claustrum individuals from binge consumption of drugs. Thus, claustrum-deficient mice consumed much more opioids than did control mice, and did so in much greater binges.
We propose that the HOMECAGE can serve as a valuable tool for relevant studies on oral opioid intake under conditions that more closely mimic the human condition. Our tool can enable a naturalistic investigation of factors contributing to opioid addiction-related behaviors and can be used to identify novel treatments.
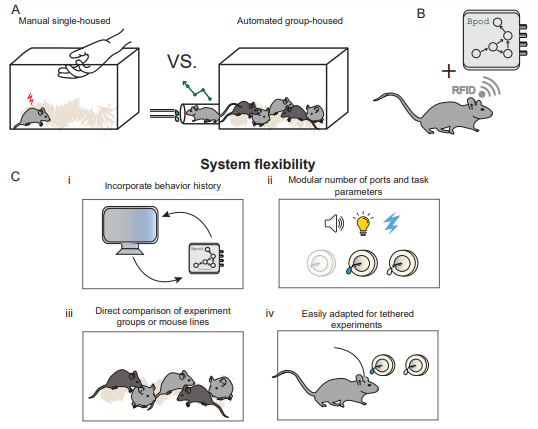
Figure 1: Overview of HOMECAGE, an automated group-housed oral self-administration procedure. (A) Most rodent studies of drug consumption or self-administration are performed on single-housed rodents, involving manual training by the experimenter. In this manuscript, we describe the HOMECAGE procedure that allows automated, individually tailored, group-housed training. (B) HOMECAGE is based on the integration of an RFID chip for individual identification of participating subjects with the Sanworks Bpod state-machine, in the context of a home cage housing multiple mice. (C) This system affords multiple benefits, including: (i) tailoring the individual task, online, according to recent behavioral history; (ii) tailoring the task parameters and the number of ports; (iii) comparing experimental groups that are co-housed; and (iv) adaptating to tethered settings for optical recordings and/or manipulations.


