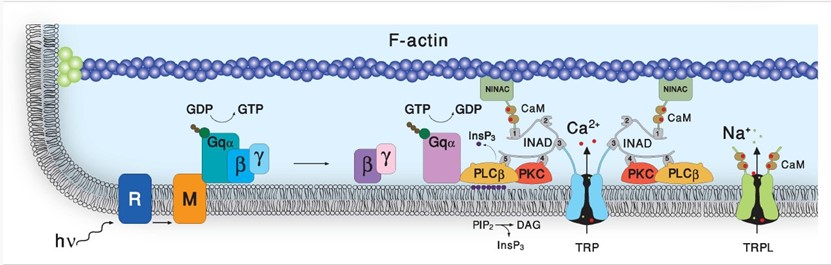
We are interested in phototransduction, the process in which light quanta are converted to electrical signals in photoreceptor cells that elicits visual sensation. To this end we use the genetically amenable biological model, the fruit fly Drosophila. We previously discovered that unlike the electronic device known as photocell, which converts light quanta into electrical current via the simple photoelectric effect, Drosophila phototransduction employed a complex enzymatic cascade composed of ubiquitous proteins common to hormonal systems (e.g. adrenaline or histamine) in which light acts as a hormone. The phototransduction enzymatic cascade includes ubiquitous signaling proteins known as G-protein-coupled to phospholipase-C-(PLC)- with Transient Receptor Potential (TRP and TRPL) as the transducer channels, whose importance in skin-sensations and pain was recently recognized by the Nobel Prize awarded to Dr. David Julius. This complex enzymatic cascade also includes regulatory enzymes such as Protein Kinase C (PKC, that regulates enzymatic activity by attaching phosphoric acids to proteins), structural proteins, such as INAD, F-actin and NINAC and cellular messengers (intracellular hormones) such as membrane bound DAG and soluble InsP3, (see Figure 1). Studies showed that sterol molecules (e.g. cholesterol) are an important component of the fatty plasma membrane that engulf all living cells including neurons, while the TRP channels reside in membrane domains called lipid-rafts. Importantly, sterol sequestration from the plasma membrane leads to the disassociation of proteins from lipid rafts and decreases the clustering of raft-associated molecules, while having no effect on another fatty domain of the plasma membrane known as PIP2-clusters, which is essential for PLC enzymatic activity (see Figure 1).
In the present study, we examined the effect of membrane sterol reduction on PLC enzymatic activity and the ensuing electrical response to light. To do so, we monitored the electrical activity of the light activated TRP channels and simultaneously monitored PLC enzymatic activity via fluorescence measurements (Figure 2). These measurements revealed that sterol reduction virtually abolished the electrical light response while having a minimal effect on PLC enzymatic activity (Figure 2). Furthermore, we found that sterol reduction suppressed a constitutively active TRP mutant-channel, suggesting that membrane sterol level determines TRP channel activity. Thus, sterol reduction prevents TRP and TRPL channels’ openings by separating light-activated PIP2-hydrolysis by PLC enzymatic activity, (which takes place in PIP2-clusters) from the TRP channels that reside in lipid rafts. Overall, our results suggest that the PLC enzyme and the TRP channels reside in separated lipid domains of the plasma membrane (i.e., lipid-rafts and PIP2-clusters) while their interaction is required for generating the light response. Importantly, sterol reduction disrupts this interaction and inhibits channel-openings. This mechanism sheds new light on the still unclear light-activation of TRP channels.
Figure 1: The phosphoinositide cascade of vision. A diagram showing the molecular components of the phototransduction cascade of Drosophila photoreceptors: Upon absorption of a photon (hν), the visual pigment rhodopsin (R) is converted into metarhodopsin (M) leading to the activation of heterotrimeric G protein (Gqαβγ) by promoting the GDP-to-GTP exchange. The GTP-bound Gqα, in turn, leads to the activation of phospholipase Cβ (PLCβ), which hydrolyses the phospholipid PIP2 (black dots) into the soluble Inositol tris phosphate (InsP3) and the membrane-bound diacylglycerol (DAG). PLCβ activates the TRP and TRPL channels through a mechanism that is still unclear, leading to light excitation combined with an increase in cellular Ca2+ concentration. Light-induced elevation of DAG participates in light excitation and together with Ca2+ also promotes activity in the eye-specific protein kinase C (PKC), which regulates channel activity. PLCβ, PKC, and the TRP ion channel form a supramolecular complex with the scaffolding protein INAD, which is bound to the F-actin cytoskeleton via the NINAC motor protein. INAD is important for keeping the stoichiometry among the signaling proteins.
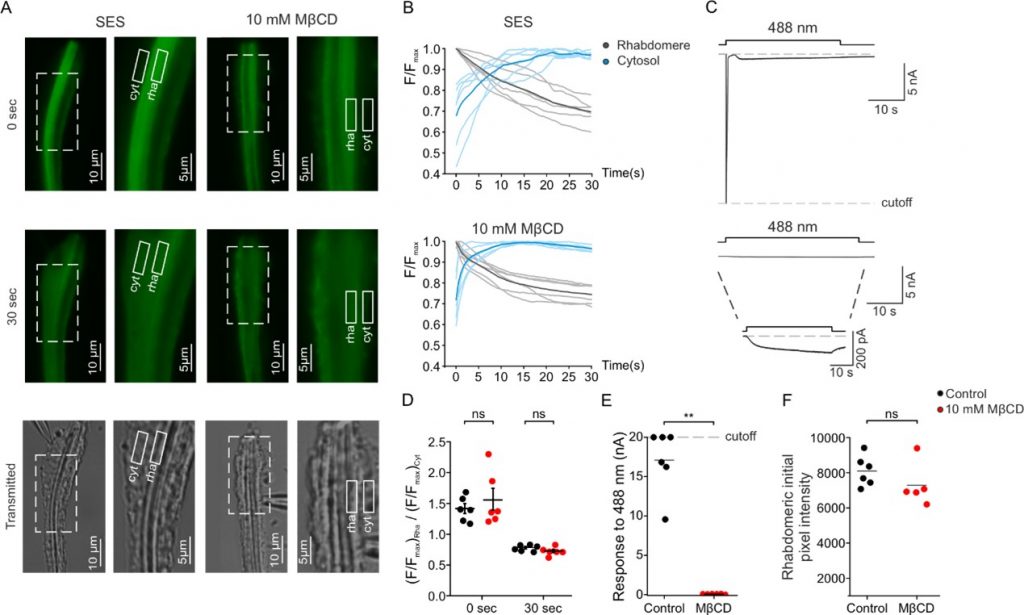
Figure 2: Sterol reduction virtually abolished the electrical light response while having a minimal effect on the light-activated PIP2-hydrolysis by PLC.
PLC enzymatic activity was measured as a function of time in the photoreceptor cells using a transgenic Drosophila fly expressing a fluorescent-tagged probe that binds to PIP2 in the photoreceptor’s signaling domain called rhabdomere (rha, shown schematically in Figure 1) and stop fluorescing upon PIP2 hydrolysis. Two fluorescent images in fixed time after excitation (0, 30 seconds) are shown in panel A and continuous fluorescent measurements are shown in panel B. White-drawn boxes indicate cellular regions where the fluorescence was measured (rha, cell body (cyt)) The non-fluorescing bottom images show transmission microscopic images of the same cellular regions. Sterol reduction was obtained by the incubation of photoreceptors with a cyclic oligosaccharide (MβCD) that sequester sterols. Panels A and B show that MβCD had virtually no effect on PLC hydrolyzing activity. Strikingly, the incubation with MβCD virtually abolished the electrical light-response when compared to incubation with Standard Extracellular Solution (SES upper part of panel C, compared to lower part of panel C) that was measured simultaneously with the fluorescent-tagged probe (upper part of panel B, compared to lower part of panel B). Panels D, E, F present quantification of the data presented in the other panels.