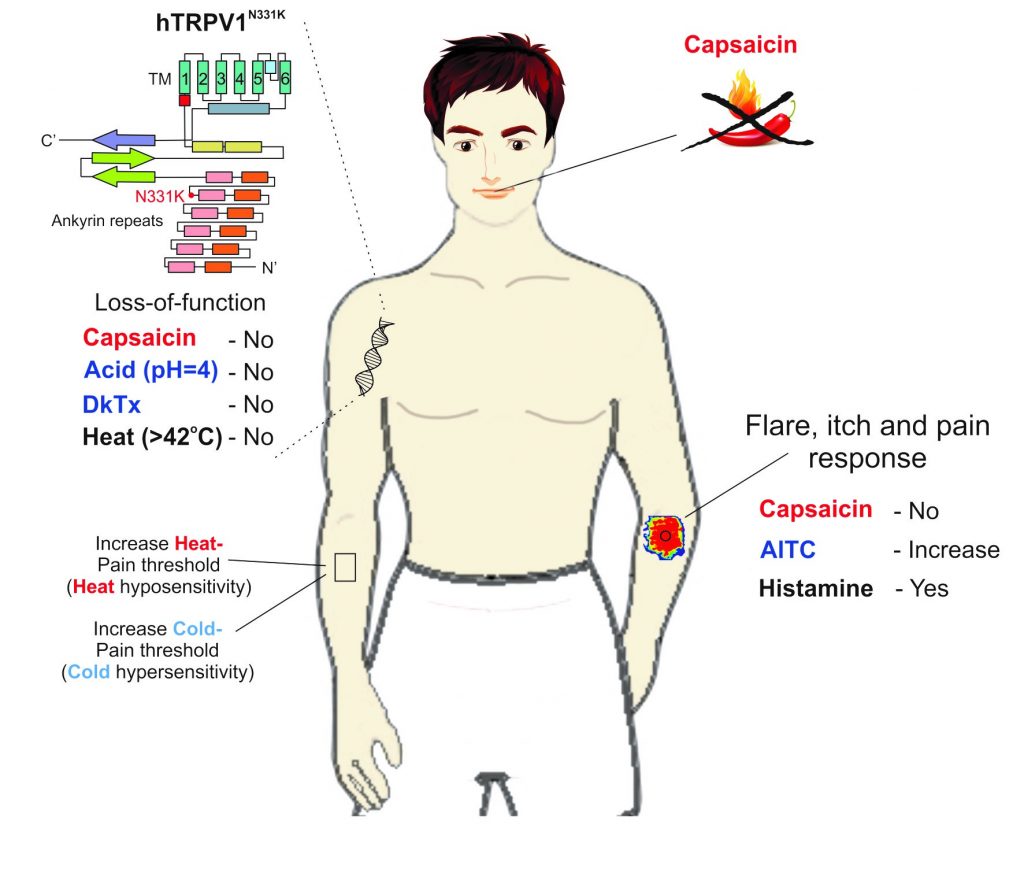
Chronic pain is a debilitating illness with currently limited therapy, in part due to difficulties in translating treatments derived from animal models to human patients. Direct understanding of pain physiology in humans could facilitate pain treatment, and the study of humans carrying genetic mutations that affect pain may promote this goal.
We studied the transient receptor-potential channel protein of TRPV1, which is associated with several body functions, specifically noxious heat-detection and inflammatory pain. Reports of adverse effects in human trials have hindered the clinical development of TRPV1 antagonists as pain relievers. Here, we examined two affected human individuals (A1 and A2) who carry a unique missense mutation in a domain of the TRPV1gene, rendering the channel nonfunctional. This conclusion was derived from experiments demonstrating the inability of different TRPV1 activators, acting at different sites of the protein, to open the channel in vitro (see Figure 1) and the insensitivity of the affected individuals to application of capsaicin (the active ingredient of chili peppers) to the mouth and skin. Biochemistry and imaging analysis in vitro suggested that the functional loss of the TRPV1 channel is not a consequence of impairment in the expression, cellular trafficking, or protein-subunits assembly. Quantitative sensory tests of A1 over the course of several years consistently demonstrated an elevated heat-pain threshold and lack of aversion towards capsaicin. Surprisingly, A1 showed an elevated cold-pain threshold and an extensive neurogenic inflammatory flare and pain-responses following application of mustard oil, a TRPA1 channel activator (see Figure 2). Our study provides direct evidence for pain-related functional changes linked to TRPV1 in humans, and could be a prime target in the development of pain relievers.
Figure 1:
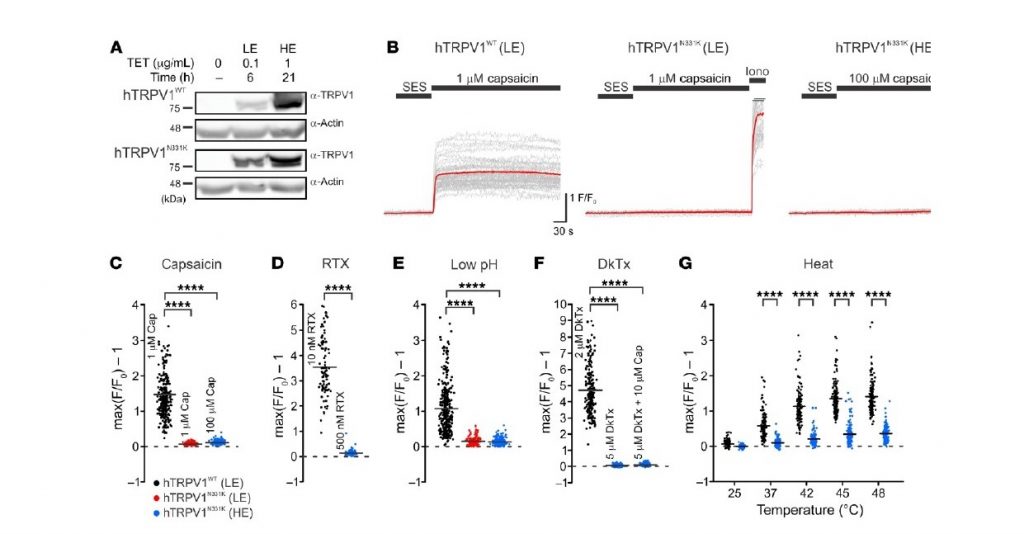
Figure 2: