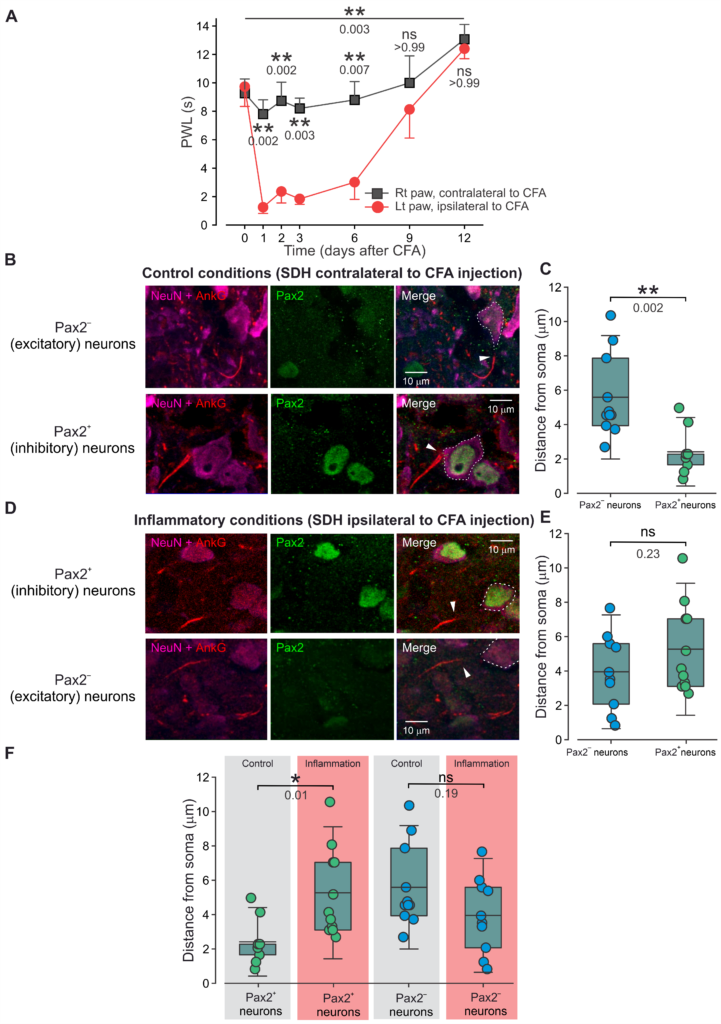
The structural plasticity of the axon initial segment (AIS), the neuronal site at which synaptic inputs are converted into an action potential (AP) output, is a pivotal mechanism underlying changes in neuronal excitability in various pathological conditions such as type 2 diabetes, Alzheimer’s disease, and stroke. In this paper, Caspi et al. show the first evidence of inflammation-induced AIS plasticity in the central nervous system (CNS) that underlies inflammatory hyperalgesia and pain. Moreover, they demonstrate that in pathological pain, the AIS plasticity-mediated network effect might be different from the expected effect in other neural systems.
Previous studies showed that the increase in input leads to AIS plasticity, resulting in a decrease in neuronal excitability and a tuning down the network’s output. Caspi et al. show that increased input indeed triggers AIS plasticity and results in a decrease in neuronal excitability. However, since it occurs selectively in the inhibitory neurons, the resulting overall network output would be increased. Overall, their results are striking in that they demonstrate that inflammation-mediated enhanced input from primary nociceptive neurons can trigger differential changes in the intrinsic excitability of CNS neurons, thus tuning the overall neuronal network response to a signal about an ongoing injury. The AIS plasticity, which occurs in a specific neuronal type within the network, provides a novel insight into the effect of this plasticity on network activity.
Figure 1:

Figure 2: